Heat of Reaction Formula
ΔH heat change1000 moles. The formula for the heat of reaction is.
Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial
H E PV.

. Standard enthalpy of hydrogenation is defined as the enthalpy change observed when one mole of an unsaturated compound reacts with an excess of hydrogen to become fully saturated. The the heat released or absorbed the heat change q in joules J for the reaction is calculated. Ie qcal qrxn.
Heat change mass specific heat capacity temperature change. H c is the heat transfer coefficient. Th final calorimeter temperature at end.
H H 1 H 2 04 t h 250 H heat of hydration of ignited cement kJkg H 1 heat of solution of dry cement H 2 heat of solution of a partially hydrated sample and. Heat of formation of reactants 1mol of Mg02mol. To answer this question again you will need to remember that bond formation releasees energy it is a favorable.
The heat of reaction also known as Enthalpy of Reaction is the difference in the enthalpy value of a chemical reaction under constant pressure. The addition of a sodium ion to a chloride ion to form sodium chloride is an example of a reaction you can calculate this way. Standard conditions refer to the following.
Cus 4HNO 3 aq CuNO 3 2 aq 2H 2 Ol 2NO 2 g Heat of Reaction Formula. Calculate the heat of the reaction. The determination of the heat of reaction requires the knowledge of the overall heat flow balance including the heat flow through the.
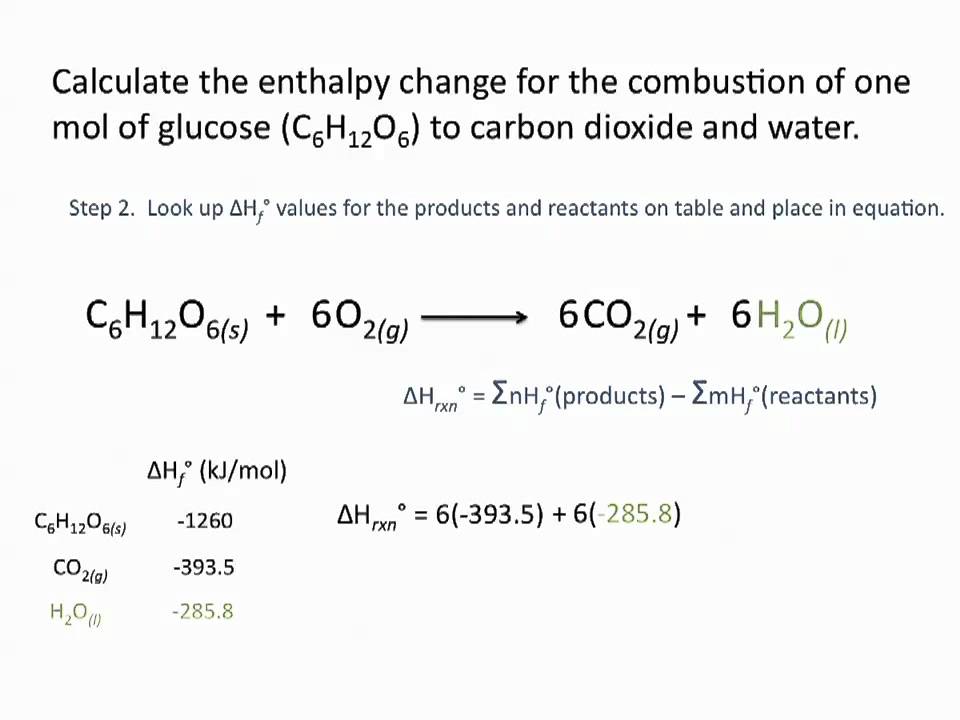
The is the heat of formation and it refers to the heat it takes to form the substance from its elements. Heat of reaction H products H reactants Heat of reaction H p r o d u c t s H r e a c t a n t s Here ΔH represents the change in heat value. The heat of reaction can be calculated based on the standard heat of formation of all reactants involved.
Solved Examples on Enthalpy Formula. Negative heat of reaction means that combustion reaction releases -20439kJ of energy. In this case the chemical reaction or physical process is endothermic.
The hydrogenation of one mole of acetylene yields ethane as a product and is described by the equation C 2 H 2 g 2 H 2 g C 2 H 6 g. Calculate the heat of the following reaction using the table of values. This reactions chemical equation is as follows.
A Temperature is 25C or 298K. B Pressure is one atmospheric pressure or 1013 kPa. Also we have the equation as.
Check Important Notes for Unsaturated. Mg2HClMgCl 2 H 2. For example why would the sum of the ΔHbonds broken be positive and ΔHbonds formed be negative in the formula for heat of the reaction.
The formula is. The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants. If you know these quantities use the following formula to work out the overall change.
A is the area of the surface. One of the most useful kinds of heats of reaction to measure and tabulate is the. As a result the heat of a chemical reaction may be defined as the heat released into.
Whether under standard conditions or not the heats of reactions can often be measured using an apparatus called a calorimeter. Enthalpy of reaction or Heat of reaction is the heat change when the number of moles of reactants as shown in the chemical equation reacts in standard conditions to form products in standard conditions. It is the thermodynamic unit of measurement used to determine the total amount of energy produced or released per mole in a reaction.
Has given an equal amount of heat to the reaction or process. Q HcA THot-TCold Where Q is heat transferred through convection. A common question when working with enthalpy energy heat and thermodynamics in general is the issue with signs.
Q m c g ΔT. The Heat of Reaction also known as enthalpy of. However it is usually determined by measuring the heat production over time using a reaction calorimeter such as a heat flow calorimeter.
Reactants in their usual physical states the measured heat of a reaction is called the standard enthalpy given the symbol ΔHo. Consider the reaction of dissolving a piece of copper in concentrated nitric acid which generates a gas. The formula for heat transferred by the process of convection is expressed as.
So it is an exothermic reaction. The reaction takes place in a closed system with a moveable piston that keeps the pressure constant. In general then the heat change for the calorimeter is equal but opposite in sign to the heat change for the chemical reaction or physical process taking place in it.
This is the combustion reaction of propane. The have values of 0 because they are in elemental form. The enthalpy change ΔH in kJ per mole of a given reactant for the reaction is calculated.
T Cold is the temperature of the cold system. H Hproducts Hreactants. T Hot is the temperature of the hot system.
In symbols the enthalpy H equals the sum of the internal energy E and the product of the pressure P and volume V of the system. C denotes the specific heat capacity of the medium ΔT denotes the difference in temperature of the medium.

Chemistry 10 5 Heat Of Reaction Chemistry 10 Chemistry Chemical Equation

Hess S Law Chemical Changes Chemistry Physical Chemistry

Chemical Reactions Chemical Reactions Occur In Predictable Patterns Chemical Reactions Chemical Changes Chemical
No comments for "Heat of Reaction Formula"
Post a Comment